Biosorption of Zn (II) ion from Aqueous Solutions Using Nut Grass
DOI:
https://doi.org/10.5530/ctbp.2024.2.25Keywords:
Biosorption, Nut grass, isotherms, kinetics, ZincAbstract
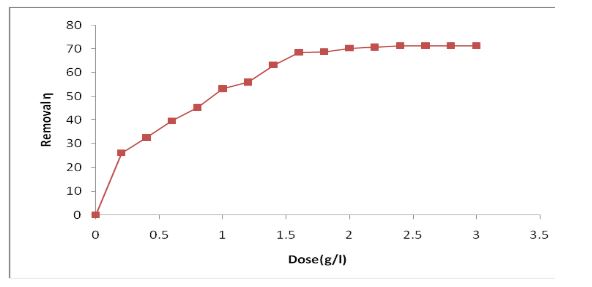
The present investigation, removal of Zn (II) ion from industry effluent was examined by using Nut grass as a biomass. The process conditions of agitation time and dosage were estimated using batch biosorption. The equilibrium data analyzed with Freundlich and Langmuir isotherms. The results revealed that removal of Zn (II) using Nut grass well followed to the Freundlich model than Langmuir model. The first order and pseudo- second order kinetic expressions were tested to find the best fit for the given kinetic data. It was observed that second order kinetics was the best fit for biosorption of Zn (II) ion. The maximum biosorption capacity of the nut grass for biosorption of Zn (II) ion was occurred at 40 mg/g.