A New Validated Stability Indicating RP-HPLC Method for Estimation of Vorasidenib in Bulk and in its Pharmaceutical Dosage Form
DOI:
https://doi.org/10.5530/ctbp.2025.2s.6Keywords:
RP-HPLC, PDA Detector, Vorasidenib, Method ValidationAbstract
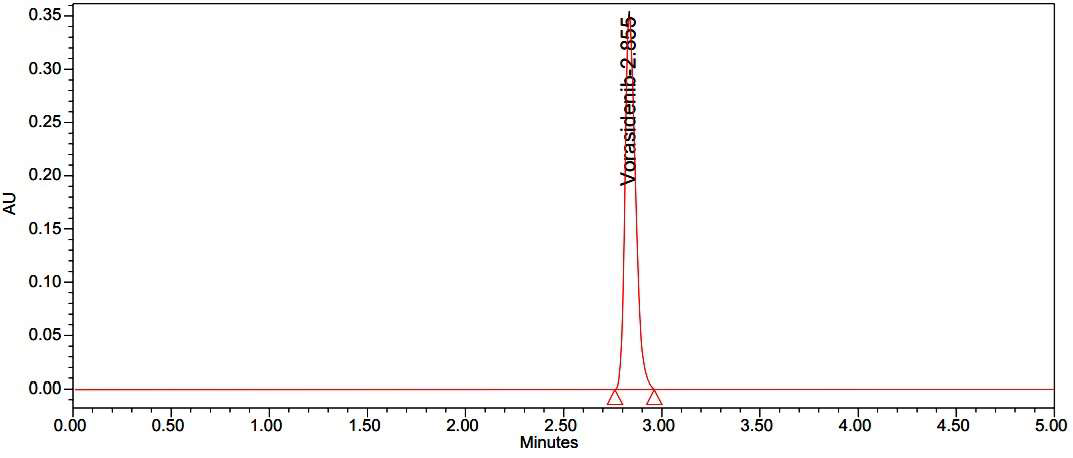
For the estimation of Vorasidenib in bulk and in its pharmaceutical dosage form, a reversed phase high-performance liquid chromatographic approach has been designed and validated in the current work. The separation of Vorasidenib was achieved on Waters Alliance-e2695, by using an XBridge Phenyl column (250x4.6mm, 5μ) by eluting with a mobile phase consisting of a mixture of acetonitrile and 0.1% Trifluoroacetic acid in the ratio of 40:60v/vat a flow rate of 1.0 mL/min; detection was carried out by absorption at 234 nm using a photodiode array detector at ambient temperature. The total run time set for the elution of the compound was 5 min. Under the optimised chromatographic conditions, the retention time was obtained at 2.855 min. The current analytical technique validation was conducted in accordance with ICH standards (ICH, Q2R1). The concentration range forVorasidenib in the linearity study was found to be 20–120 μg/mL and the coefficient of variance was found to be 0.9999. The percentage recovery was found to be 99.6-100.3%. LOD and LOQ were found to be 0.48 μg/mL and 1.6 μg/mL respectively. The developed method was also applied to monitor the forced degradation studies on the drug for testing for its ability to resolve the drug from their degradation products. The specificity of the developed method was evaluated by applying acid, base, oxidation, thermal, photolytic and neutral stress conditions to the drug. It was concluded that the estimation of Vorasidenib in bulk and its pharmaceutical dosage form was found to be successfully conducted by using the method.