Development and Validation of Bio-Analytical Method for Simultaneous Estimation of Metformin, Vildagliptin and Remogliflozin in Rabbit Plasma by using RP-HPLC
DOI:
https://doi.org/10.5530/ctbp.2025.2s.7Keywords:
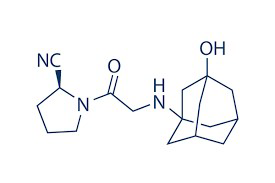
Metformin, Vildagliptin, Remogliflozin, Saxagliptin, ValidationAbstract
The current investigation was intended to develop and validate bio-analytical RP-HPLC method for the simultaneous analysis of Metformin, Vildagliptin and Remogliflozin in rabbit plasma employing Saxagliptin as an internal standard. Protein precipitation method was used to extract the analytes from spiked plasma samples. Chromatographic separation of extracted analytes was achieved on X Bridge C18, (150 mm X 4.6 mm and 3.5 μm) column using the mobile phase consists of 0.01N KH2PO4: acetonitrile (70: 30 v/v, pH 5.4). Isobestic point of 235 nm wavelength was selected for quantification of drugs. The peaks eluted at 2.262 min, 3.850 min and 5.903 min were recognized as Metformin, Vildagliptin and Remogliflozin respectively. The present method showed desirable proportional response in the range of 25-2500 ng/mL for Metformin, 5-1000 ng/mL for Vildagliptin and 25-2500 ng/mL for Remogliflozin. Low variance was observed (%CV) in the results of precision and accuracy and excellent and reproducible recoveries were obtained with spiked plasma samples. Stability studies such as long term, short term and freeze thaw stability were performed and produced inconsistent results. The results revealed the proposed method can be appropriate for the simultaneous bio-analysis of Metformin, Vildagliptin and Remogliflozin in rabbit plasma and successfully employed for pharmacokinetic studies.