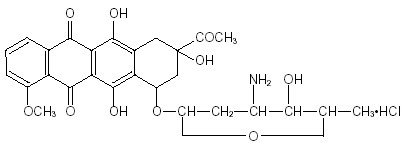
A Novel RP-UPLC Technique for Quantification of Daunorubicin and Cytarabine Simultaneous in Combined Tablet Dosage Forms - Its Application in Monitoring Forced Degradation Studies
DOI:
https://doi.org/10.5530/ctbp.2025.2s.10Keywords:
RP-UPLC, Daunorubicin, Cytarabine, Simultaneous quantification, Forced degradation, Stability-indicating methodAbstract
To establish and evaluate new reverse-phase ultra-performance liquid chromatography (RP-UPLC) technique to measure daunorubicin and cytarabine at the same time in combined tablet forms and to use this technique to monitor forced degradation studies. Using standardized testing, the authors identified a technique, reverse-phase ultraperformance liquid chromatography (RP-UPLC), on a piriform C18 column, 50mmx2.1mm OD core particle sizes, 1.7 micron. This approach used a changing mix of acetonitrile and a phosphate buffer with a pH level of 3.0. The liquid moved through the system at a rate of 0.3mL per minute, and UVlight detected substances at a wavelength of 254 nm. For the robustness of the method to be ensured, the scientific (research) community has used the ICH guidelines, has estimated the following functionalities, i.e., repeatability, reproducibility, accuracy, robustness and reliability. Moreover, the investigators also proved the protocol's versatility in various stress environments (acidic and basic stress, oxidative stress and thermal stress, light exposure and radiation). The method exhibited excellent linearity for both daunorubicin and cytarabine of concentration of 0.5–50μg/mL (R² > 0.999). Forced degradation studies demonstrated efficient separation of the analytes and degradation productsof the corresponding analytes, confirming the stability-indicating capability of this technique. Validation results indicated high precision, accuracy, and robustness, making the method suitable for routine quality control.Thus, this newly established RPUPLC techniqueis an efficient and reliable procedure for the quantification of both the drugs at the same timein combined formulation.It is also a strong stabilityindicating method forforced degradation studies ensuring the quality and purity of the pharmaceutical formulation.