Formulation and Evaluation of Tinospora Cordifolia - Cholesterol Phytophospholipid Complex for Enhanced Anti-Inflammatory Activity
DOI:
https://doi.org/10.5530/ctbp.2025.2s.11Keywords:
Tinospora cordifolia, Phytophospholipid, Anti-inflammatory activity, Sustained release, Drug entrapmentAbstract
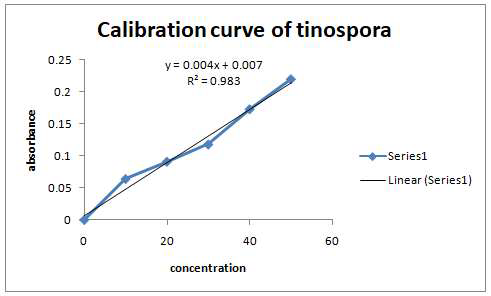
Tinospora cordifolia is widely recognized for its anti-inflammatory and immunomodulatory properties, offering therapeutic potential for managing inflammatory disorders such as arthritis and chronic autoimmune diseases. To develop and evaluate a Tinospora cordifoliacholesterol phytophospholipid complex to enhance entrapment efficiency and optimize drug release profiles, facilitating its application in inflammatory disorder management. To compare the drug entrapment efficiency and release profiles of nine formulations, identify the impact of cholesterol-to-extract ratios, and determine the most effective formulation. Nine formulations (F1–F9) of the Tinospora cordifolia-cholesterol phytophospholipid complex were prepared and evaluated for drug entrapment efficiency and in vitro release over eight hours. Burst and sustained release characteristics were analyzed, and the influence of varying cholesterol ratios was assessed to determine optimal formulation parameters. Formulation F7 showed the highest entrapment efficiency (92.91%), followed closely by F4 (92.31%), both indicating superior drug retention. F4 displayed an optimal release profile, with a burst release of 36.9% in the first hour and sustained release of 92.3% by the eighth hour. Higher cholesterol ratios, as seen in F4 and F2, enhanced stability and controlled release, while lower ratios in formulations such as F1 and F7 resulted in reduced performance. This research demonstrates the feasibility of a cholesterol-based phytophospholipid complex for enhancing the delivery of Tinospora cordifolia. Formulation F4 emerged as the most promising candidate due to its balanced burst and sustained release properties, offering potential for clinical applications in inflammatory disorder management. Future studies should validate its pharmacokinetic and in vivo therapeutic efficacy.