Bioanalytically Validated LC-UV Method for Estimation of Hypoxia-Inducible Factor-2 alpha (HIF-2α) Inhibitor in Spiked Human Plasma
DOI:
https://doi.org/10.5530/ctbp.2025.2s.12Keywords:
Belzutifan, Recovery, Precision, Therapeutic drug monitoringAbstract
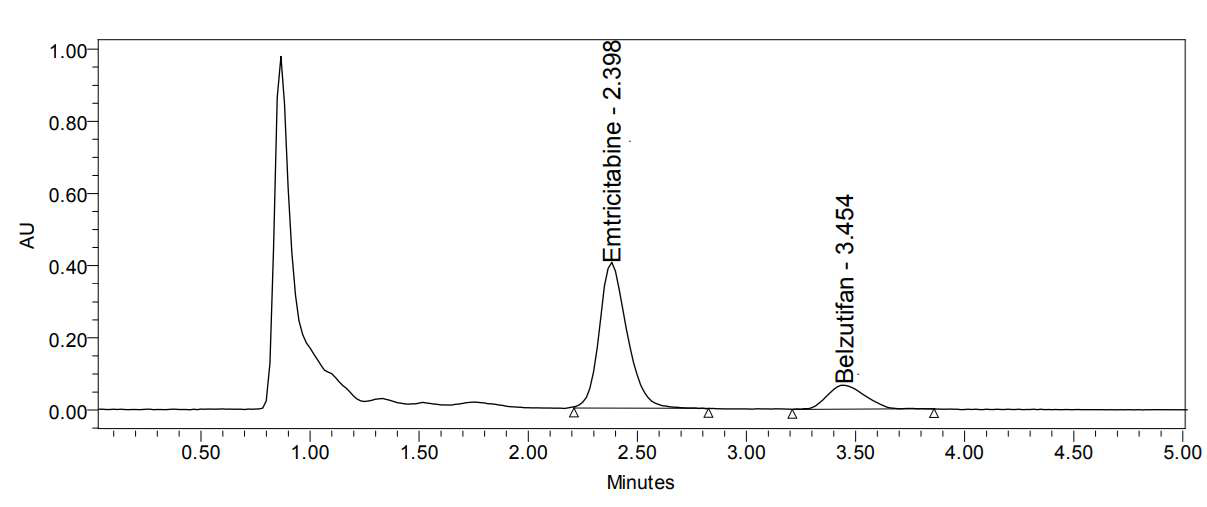
Belzutifan is a hypoxia-inducible factor- 2 alpha (HIF-2α) inhibitor used to treat von Hippel–Lindau disease-associated renal cell carcinoma. Simple, accurate, precise, and specific HPLC method was developed to estimate Belzutifan (BZT) in human plasma serving Emtricitabine (ETC) as reference (ISTD). The analyte and ISTD were separated on a Kromasil C18 (250x4.6mmx5μ) column using a mobile phase composition. The buffer was composed of Acetonitrile (60:40). The RTs of the analyte and ISTD were found to be 3.446 and 2.363 min, respectively with a flow of 1ml/min. Further, the reported method validated as per USFDA, and was found to be well within the acceptable range for all parameters with concentrations of LLOQ 0.075mcg/ml, LQC 0.225mcg/ml, MQC 1.5mcg/ml, HQC 2.4mcg/ml, and ULOQ 3.0 mcg/ml. The matrix effect at HQC and LQC was 100.19 and 99.83%; the sensitivity at LLOQ was 99.63%; the precision and accuracy at HQC, MQC, LQC, and LLOQ was between 98.56 and 100.11%. The linearity concentration is in the range of 0.75-3mcg/ml Belzutifan with a correlation coefficient of r2 = 0.999 with good stability. The proposed HPLC method as easy, fast, and particular for the calculation of Belzutifan in human plasma. Efficient sample preparation and rapid chromatographic analysis are crucial for high-throughput detection and quantification of target analytes in complex matrices such biological matrices. Thus, the reported HPLC method can be applied to the bioequivalence and pharmacokinetic studies of Belzutifan in human plasma samples and is appropriate for therapeutic drug monitoring in clinical laboratories.