Bioanalytical Method Development and Validation for Quantification of an Anti-Neoplastic Agent - Glasdegib by using LC–MS/MS (ESI) in Human Plasma
DOI:
https://doi.org/10.5530/ctbp.2025.2s.15Keywords:
Glasdegib, Development, Validation, LC-MS/MS, Biological FluidAbstract
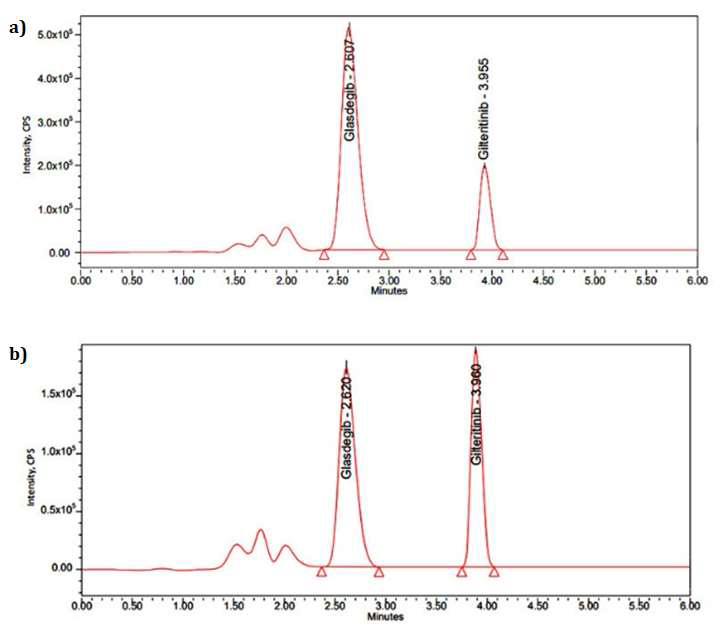
Glasdegib is inhibitor of a Hedgehog signaling pathway used in the treatment of cancer associated with Sonic Hedgehog protein overexpression like breast, pancreatic, medulloblastoma, etc. Since this drug was recently approved by the food and drug administration the effectiveness of the treatment as well as the quality control of this drug need to be monitored. The highly sensitive and selective analytical technique like LC-MS/MS is necessary to monitor the quality and quantity of this drug in biological fluids. Hence this work aimed to develop an LC-MS/MS method to accurately quantify Glasdegib in biological fluids. Quantification of this drug was achieved by using a C18 symmetric column (150 mm x 4.6 mm, 3.5 μm) and isocratic elution, with a mobile phase containing Acetonitrile: 0.1% formic acid at a 30:70 ratios. The flow rate was set at 1 mL/min, and the mobile phase pH was adjusted to 4.0.The retention time for Glasdegib was found as 2.62 min, and a linear curve was established for concentrations between 6.00 and 120 ng/mL with regression coefficient of 0.999.Results showed that system suitability parameters, including theoretical plates, tailing factor, and resolution, within acceptable limits. Recovery testing indicated 99.94% extraction efficiency, while matrix effect studies revealed minimal interference (98.56%). Validation results of accuracy, linearity, and LOD/LOQ were found within acceptable ranges.The proposed LC-MS/MS method provides a sensitive, accurate, and reliable analytical approach for measuring Glasdegib in biological matrices, supporting its clinical applications in cancer treatment.