Reversed Phase UPLC Method Estimation of Lovastatinin Novel Hydrogel Formulation: Application of method to Pharmacokinetics, Pharmacodynamics and Histopathological samples
DOI:
https://doi.org/10.5530/ctbp.2025.4s.13Keywords:
RP-UPLC, Validation, Hydrogel, Lovastatin, Pharmacokinetics, PharmacodynamicsAbstract
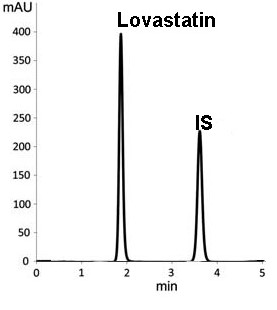
Lovastatin is used to treat high blood cholesterol and reduce the risk ofcardiovascular disease. In this study, an ultra-high-performance liquid chromatography (UPLC) method was developed to measure the concentrations of Lovastatin in rat blood, and the method was applied in measuring the pharmacokinetics of the analyte after oral and intravenous administration. The analyte was extracted by solid phase extraction method. A. UPLC BEH C18 column (2.1 mm x 100 mm, 1.8 µm particle size) was used for chromatographic separation by gradient elution using acetonitrile-water (0.1% formic acid) as the mobile phase at a flow rate of 0.4 mL/min. Lovastatin was administered to the rats orally at 2 mg/kg and intravenously at 0.05 mg/kg. Blood was collected at various time intervals, and the blood samples were processed after collection and analyzed by UPLC. The intra-day and inter-day accuracy of Lovastatin were 91%-103% and 85%-107%, respectively, and the precision (RSD, %) was less than 15% for both intra-day and inter-day measurements. The matrix effect ranged from 95% to 108%, and the recovery was higher than 70%. Lovastatin has a good linear relationship in the range of 10-500 ng/mL, and the lower limit of quantification was 10 ng/mL. The precision, accuracy, extraction recovery, matrix effect, and stability meet the requirements of the guiding principles. A robust and reliable UPLC method was fully optimized and developed to detect the blood concentration of Lovastatin in rats and the samples were analyzed by Empower software.