Review on Quality by Design Approach (Qbd)
DOI:
https://doi.org/10.5530/ctbp.2021.4.47Keywords:
NilAbstract
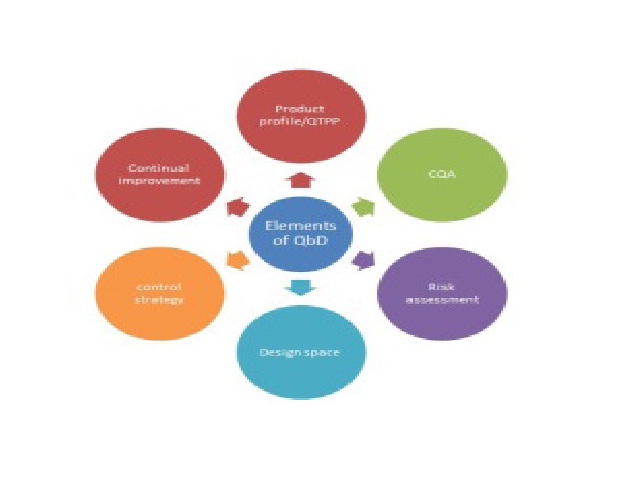
In pharma world QbD is one of the utmost influential tactic to confirm quality of the pharmaceuticals. By means of regulatory aspects QbD is based on ICH guidelines Q8, Q9, Q10 (where as Q8 stands for pharmaceutical development, Q9 stands for quality risk management and Q10 stands for pharmaceutical quality systems). In this review it explains how QbD can be achieved, what are the benefits and drawbacks of QbD and the modules, software’s and the experiments conducted based on QbD are explained and it also elucidates about the QbD tools (such as risk assessment) in order to lessen the errors in the process to assure the quality of the pharmaceuticals.