Formulation and Evaluation of Glibenclamide by Solid Dispersion Method using Compritol 888 ATO as a Carrier
DOI:
https://doi.org/10.5530/ctbp.2022.2.13Keywords:
Glibenclamide, Fast Dissolving Tablets, Synthetic polymer, Compritol 888 ATO, AntidiabeticAbstract
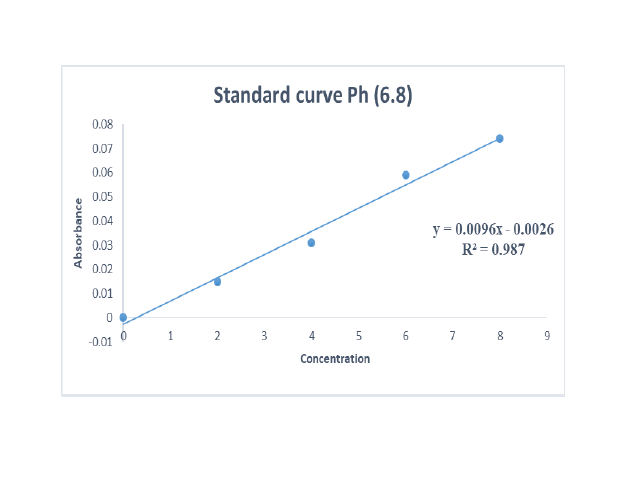
Glibenclamide is an antidiabetic drug that is poorly soluble, to enhance its rate of dissolution, it is formulated into solid dispersions using various polymers by solvent evaporation method. The present study aims to enhance the dissolution of poorly soluble drug Glibenclamide by solid dispersion technique using solvent evaporation method with various carriers such as Compritol 888 ATO, PEG 6000, at ratios (1:1,1:2 and 1:3) and to study the effects of carriers on the dissolution of the drug. The solid dispersions characterized by FTIR showed no possible interactions between drug and carriers, XRD results showed conversion of crystallinity of drug to its amorphous form. The in vitro results showed a significant enhancement in the dissolution rate of all solid dispersion prepared by solvent evaporation method when compared with the pure drug itself (39.82%). Among the two polymers, Compritol 888 ATO and PEG6000 the formulation SD9 containing Compritol 888 ATO as a carrier at 1:3 ratio showed the highest rate of dissolution (99.17%), thus the concentration of carriers used in this study played an important role in enhancing dissolution. This study concludes that Glibenclamide’s dissolution rate can be significantly increased using Compritol 888 ATO and PEG6000 as polymers by solvent evaporation and lyophilization method, from which Compritol 888 was chosen as the best polymer for enhancing in-vitro dissolution rate of poorly soluble drug Glibenclamide.Keywords: Glibenclamide, Fast Dissolving Tablets, Synthetic polymer, Compritol 888 ATO, Antidiabetic